Whether it’s for your child’s toy, your cordless power tool, or your electric vehicle, battery power is becoming a standard part of our everyday lives. One of the most critical components of a battery is the internal battery electrolyte.
Today we’re looking at what battery electrolyte is and how it keeps your battery-powered life running. Let’s dive in!

What Is Battery Electrolyte?
The battery electrolyte is a solution inside batteries. Depending on the type of battery, it can be a liquid or paste-like substance. However, no matter the type of battery, the electrolyte serves the same purpose: it transports positively charged ions between the cathode and anode terminals.
How Does Battery Electrolyte Work?
A battery has three major components – the cathode, the anode, and an electrolyte that separates these two terminals. The electrolyte is a chemical that allows an electrical charge to pass between the two terminals. The electrolyte puts the chemicals required for the reaction in contact with the anode and cathode, therefore converting stored energy into usable electrical energy. This reaction provides power to the connected device, whether it’s a light, a vacuum, or an electric vehicle.
What Is the Battery Electrolyte Made Of?
Different types of batteries rely on different types of chemical reactions and different electrolytes. For example, a lead-acid battery usually uses sulfuric acid to create the intended reaction. Zinc-air batteries rely on oxidizing zinc with oxygen for the reaction. Potassium hydroxide is the electrolyte in common household alkaline batteries. The most common electrolyte in lithium batteries is a lithium salt solution such as lithium hexafluorophosphate (LiPF6).

If you think back to your high school chemistry class, you’ll likely remember wearing safety goggles and other protective gear when handling chemicals. Chemicals you’d use to create chemical reactions in batteries are often hazardous, so take the proper precautions when working with batteries and their electrolytes.
Can You Add Electrolyte To A Battery?
Yes, you can add electrolyte to a battery but ONLY if it’s a non-sealed wet cell battery. Checking the levels in a wet cell battery is standard maintenance that you should do regularly.

While the electrolyte contains water and sulfuric acid, you should not add anything except distilled water to your battery. When properly functioning, a wet cell battery will only consume the water.
If your battery is sealed or doesn’t consume the electrolyte in off-gassing, you can’t add electrolyte. Nor do you need to. The lack of off-gassing is one advantage of choosing AGM or lithium-ion batteries, as they require very little maintenance once installed.
What Are the Ingredients in Lithium Batteries?
The ingredients of lithium battery electrolytes depend on the chemistry that creates the reaction and the type of lithium battery. Most lithium batteries use a liquid electrolyte such as LiPF6, LiBF4, or LiClO4, in an organic solvent.

However, recent advances have made solid ceramic electrolytes–such as lithium metal oxides an option for batteries. The main advantage of solid electrolytes is that they eliminate the risk of leaking and eliminate flammability, which is a safety risk in batteries with liquid electrolytes.
Lithium hexafluorophosphate (LiPF6) is the most common lithium salt in lithium-ion batteries. This solution creates an incredibly stable environment for the lithium ions during charging and discharging.
How Lithium Batteries Work
Lithium-ion batteries use charged lithium ions to create an electrical potential between the anode and cathode terminals. A thin layer of insulating material called a “separator” sits in the electrolyte solution between the two sides of the battery. The separator allows the lithium ions to pass through while blocking the electrons and keeping the two electrodes apart. During charging, lithium ions move through the separator from the positive side to the negative. While discharging, the ions move in the opposite direction.
The movement of the lithium ions creates an electrical potential difference called “voltage.” When you hook your electronic devices up to the battery, electrons (not lithium-ions) flow through your device and power it.
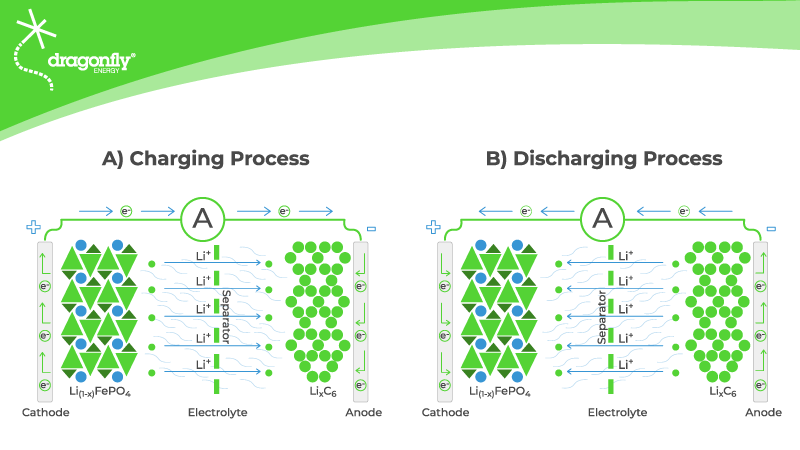
Is Lithium Battery Electrolyte Safe?
The electrolytes in lithium batteries are safe. However, in the early days of lithium batteries, thermal runaway, when the batteries caught fire, was a more prevalent issue. However, the fires were mainly due to solvents in the lithium cells overheating, getting punctured, or overcharging.
As technology advances, new options are becoming available for improving the safety of lithium batteries. For example, Battle Born’s proprietary battery management system (BMS) will shut down the battery cells if it detects unsafe conditions. The result is one of the safest batteries on the market.

Read about Dragonfly Energy’s technology and how we are revolutionizing advances in battery electrolytes and also the manufacturing process.
A Critical Component of Your Battery
Battery electrolyte is a critical component in all battery types, and in most cases, you’ll probably never even think about it. However, depending on the type of battery you’re using, understanding how the battery electrolyte works can help extend your battery’s life.
Luckily, when you invest in products like Battle Born’s lithium-ion batteries, there’s much less maintenance and no worries about the battery’s electrolyte.



This is certainly a great write-up. Thank you for bothering to explain all this out for all of us. It’s a great help!