How does a lithium-ion battery work? It’s a question many battery users have asked themselves when eyeing these high-quality lithium batteries that are winning over an increasing share of the RV, boat, and other deep-cycle markets. And the science behind these batteries can get complex and confusing for the average person, to say the least.
But if you’re trying to figure out how lithium-ion works, wonder no more. We’re breaking down this fantastic technology, its differences from traditional batteries, and why they’re becoming more and more popular.
What Are the Components of a Lithium-Ion Battery?
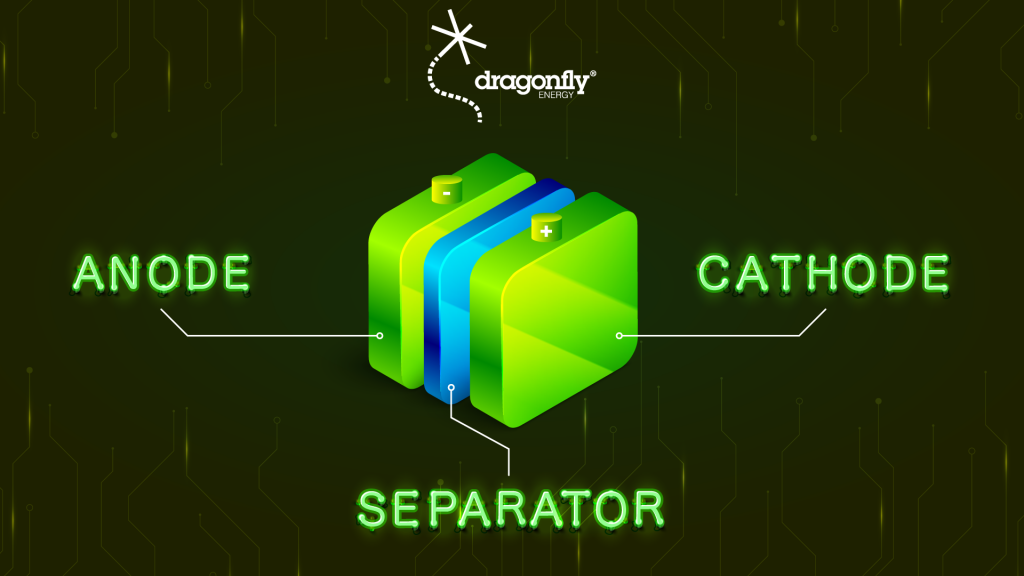
When it comes to the parts that explain how a lithium-ion battery works, it’s actually fairly simple. There are really only four essential components inside a lithium battery: the cathode, the anode, a separator, and the electrolytes. These basic components are, in many ways, the same as any other type of battery or electrochemical cell. With these four simple pieces, batteries can harness an incredible amount of lithium energy.
The difference in lithium batteries is the chemical composition of these different components.
How Is the Composition of the Cathode and Anode Different?
The chemistry of a lithium-ion battery requires different materials on the positive and negative sides of the battery.
The positively charged cathode is essentially aluminum foil coated in a lithium compound, like lithium iron phosphate (sometimes referred to as LiFePO4).
The negatively charged anode is similar in design but made with different materials. Typically, this is copper foil coated in graphite. These weren’t chosen by accident. All the compounds involved here play crucial roles in battery chemistry.
Dive Deeper: Anode vs. Cathode: What’s the Difference?
What Happens When You Charge a Lithium-Ion Battery for the First Time?
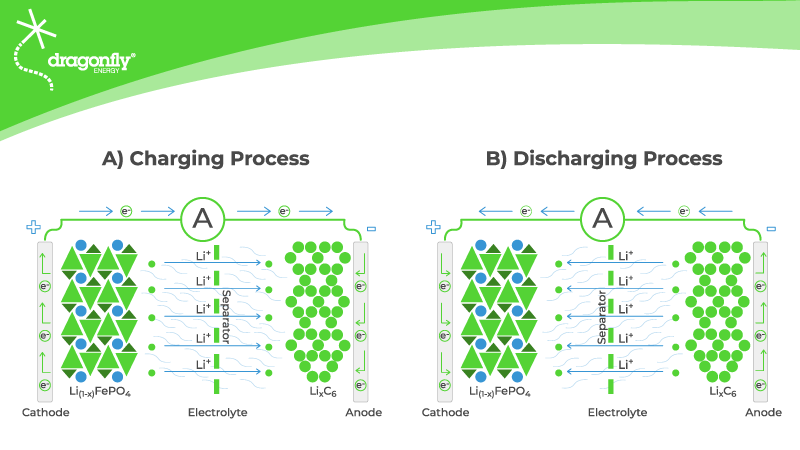
Electricity is all about electrons, and their movement is the key to the crucial changes that occur when you charge a battery. The very first charge of a lithium-ion battery is usually done by the manufacturer because of the lithium in the electrolyte.
When the battery is connected to a charger, a chemical reaction takes place involving the LiFePO4 on the cathode. This chemical reaction causes the compound to split into electrons, positively charged lithium ions, and an iron phosphate remainder.
The electrons flow to the anode through the charger. Meanwhile, the positively charged lithium ions migrate through the electrolyte to combine with the graphite on the anode. Finally, they meet again and form a compound known as lithiated graphite. Eventually, all of the lithium ions and electrons have completed this movement, resulting in a fully charged lithium-ion battery.
What Is the Difference between the Composition of a Lead-Acid Battery and the Composition of a Lithium-Ion Battery?
When answering how does a lithium-ion battery work, it can be helpful to distinguish it from old-school lead-acid batteries. As opposed to the aluminum/lithium cathode and copper/graphite anode of lithium-ion batteries, lead-acid batteries have cathodes and anodes both made of lead sulfate (PbSO4). Lead-acid batteries also use sulfuric acid as their electrolyte (H2SO4) instead of the lithium solution used in lithium-ion batteries.
Lead acid batteries use ions for transfer through the acid solution as well, but they are hydrogen ions. In a way, we could call these batteries lead hydrogen ion batteries. Because of the acid, hydrogen, and toxic lead, these batteries tend to be much more dangerous and hazardous to human health.
How Does a Lithium-Ion Battery Work Differently Than a Lead-Acid Battery?
For those without an interest in chemistry or electronics, these differences might seem technical or abstract. But they manifest in significant ways when these batteries operate. Here are a few of the most notable.
Electrolyte Composition
As noted above, lead-acid batteries use a sulfuric acid solution to serve as the electrolyte within the battery cell. Unfortunately, sulfuric acid is highly corrosive and can be dangerous if ingested, spilled on the skin, or inhaled. Even worse, many lead-acid batteries require users to regularly open their batteries to top off the electrolyte solution as it evaporates, potentially exposing users to this harmful substance.
In contrast, the lithium solution used in lithium-ion batteries presents a far lower risk. Better yet, lithium batteries are completely sealed, meaning there’s little to no chance users will come in contact with the solution except in cases of serious battery damage.
Battery Weight
One of the most apparent differences between these battery types is weight. The reason is simple: lead is exceptionally dense, weighing a remarkable amount for its size. In contrast, lithium is light. Lithium-ion battery components are also far lighter. This can be particularly important for weight-sensitive uses like boats and RVs.
Sulfation
You may not be familiar with the concept of sulfation, but it’s a vital one to know due to how it can affect lead-acid batteries. Sulfation is the process of sulfate crystals forming on the battery electrodes, which increases battery impedance. Because of this, you’ll draw far less power from the battery, eventually forcing you to replace it.
Luckily for lithium-ion users, they won’t need to worry about sulfation. It can’t happen with lithium batteries because the necessary chemical ingredients simply aren’t present. And who wouldn’t like one fewer thing to worry about when it comes to their batteries?
What Are the Advantages of Using a Lithium-Ion Battery Over a Lead-Acid Battery?
We’ve already touched on a few of the advantages of lithium-ion batteries over lead-acid. These include less maintenance and risk of harm, lower weight, and less risk of sulfation.
But these are only a few of the many benefits of making the switch to lithium. Because lithium-ion batteries are totally sealed, you can mount them in more configurations, as well as in unvented compartments. They can also be fully discharged without damaging them, unlike lead acid.
Lithium-ion batteries also work better in extreme temperature conditions. Finally, lithium-ion batteries tend to last far longer than lead-acid ones. This means that, even with their higher price tag, lithium-ion batteries generally provide a better value over the long run.

Lead Is Dead: Understand How Lithium-Ion Batteries Work and Choose a Better Battery
Lead-acid batteries may still be common, but the trend is clear. Lead is dead. We’re now in the era of the lithium-ion battery. More and more people are thinking about lithium-ion for their batteries instead of lead-acid. With fundamentally different construction and crucial differences in operation, lithium-ion batteries command several crucial advantages in the deep-cycle market, especially for heavy energy users or those in extreme conditions.
So how does a lithium-ion battery work? Very well, for most users. This is thanks to the simple but powerful chemistry inside them that you now understand.
Learn More About Our Lithium-Ion Battery Technology
Producing lithium-ion battery cells can be a messy and expensive process. That’s why we’ve set out to develop cleaner and less wasteful processes to create lithium-ion batteries. The smaller the footprint, the better.
Here at Dragonfly Energy, we are always innovating. We have the results and processes to back it up. No smoke and mirrors – just real science and forward-thinking technology.
Learn more about our technology here.



Leave a Reply